Ataxia is a term used for a group of neurological conditions. It is a complex and rare neurological condition caused by cerebellar dysfunction due to degenerative, genetic or acquired causes. There are treatments and therapies to reduce the symptoms including motor imbalance, tremor, speech difficulties and vision impairments but in most cases, there’s no cure for ataxia.
Ataxia and clinical trials
Numerous ataxia clinical trials seek treatments for ataxia symptoms and potential cures to modify the course of the disease such as gene replacement therapy.
To be able to develop therapies, it is essential to measure the severity of the disease. To this effect, a clinical scale known as the Scale for the Assessment and Rating of Ataxia (SARA) was developed to assess a range of different impairments in cerebellar ataxia including gait, speech, sitting, movements. Ataxia symptoms can be highly variable within a single day, from day-to-day and week to week, and travel to clinical sites can be stressful and time-consuming for patients, also impacting a patient’s performance while being assessed and often leading to a missed insight of disease variability.
Due to the low number of patients and the nature of the disease itself (variable and evolutive), there are many challenges encountered when conducting clinical trials. For patients living with restricted mobility and neurological impairments, there is a strong need for patient-centric trials and innovation in clinical trial design.
Development of SARAhome
As part of its commitment to improving the patient experience of clinical trials, Aparito collaborated with the German Centre for Neurological Disease (DZNE) to digitise the SARA assessment into SARAhome and devise a means for patients to record their severity of ataxia by using a simplified and shorter SARA version in a video-recordable manner at home.
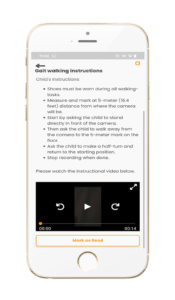
SARAhome leverages the unique capabilities of Atom5TM with its video capture and analysis to detect changes in a patient’s condition and monitor disease progression over time, a means of valuable insight with myriad applications in clinical research.
Initially designed for paediatric and rare diseases, the Atom5TM platform can enhance compliance and the potential to improve the outcomes of any clinical trial. Its data-driven, decentralised platform allows for clinical studies to be done anywhere, anytime allowing patients and their caregivers to participate in research from the comfort of their own homes.
Our success stories
Our Credentials

21 CFR Part 11 compliant

Cyber Essentials Plus

ISO 13485

ISO 27001

ePrivacy App





